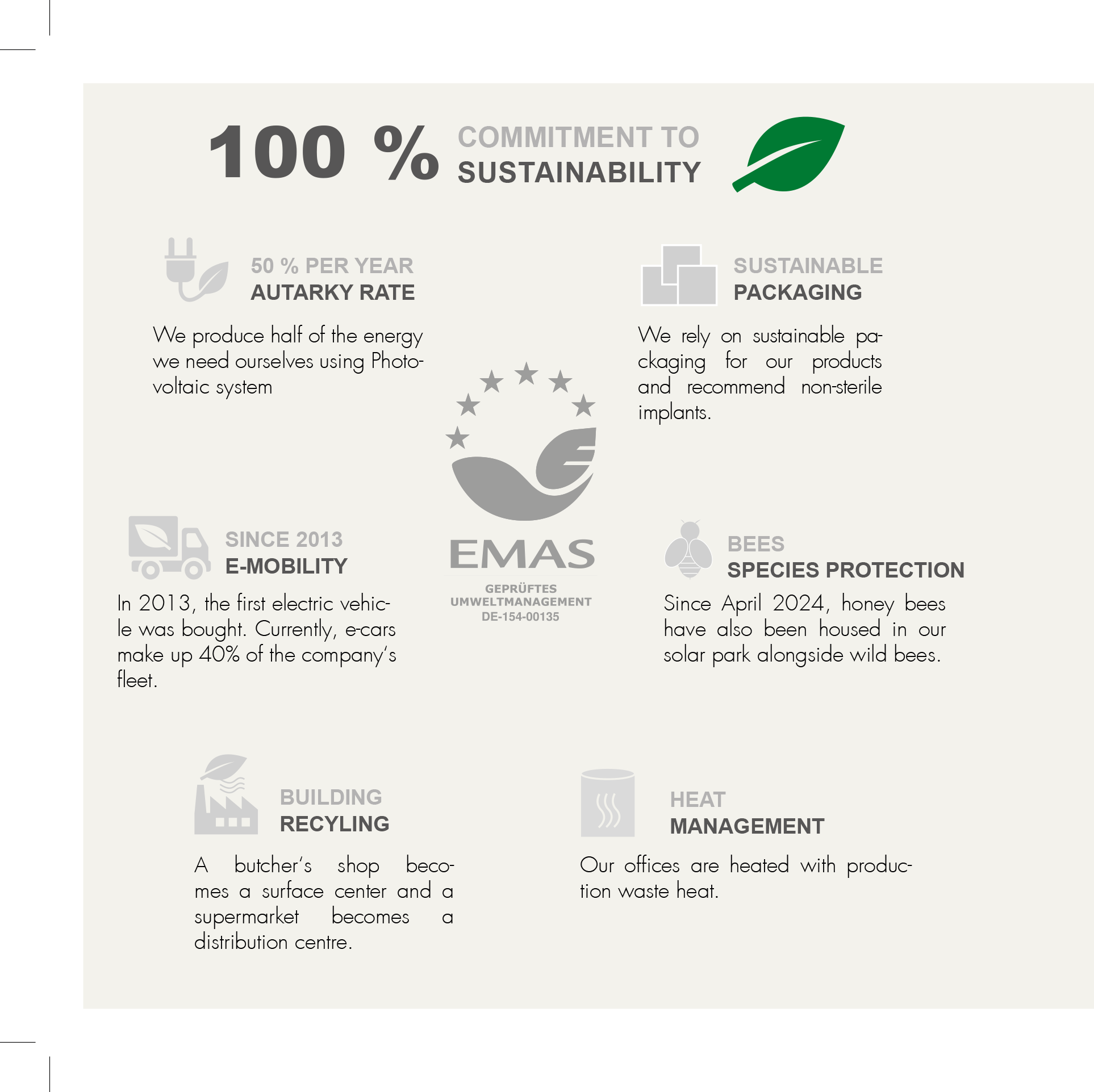
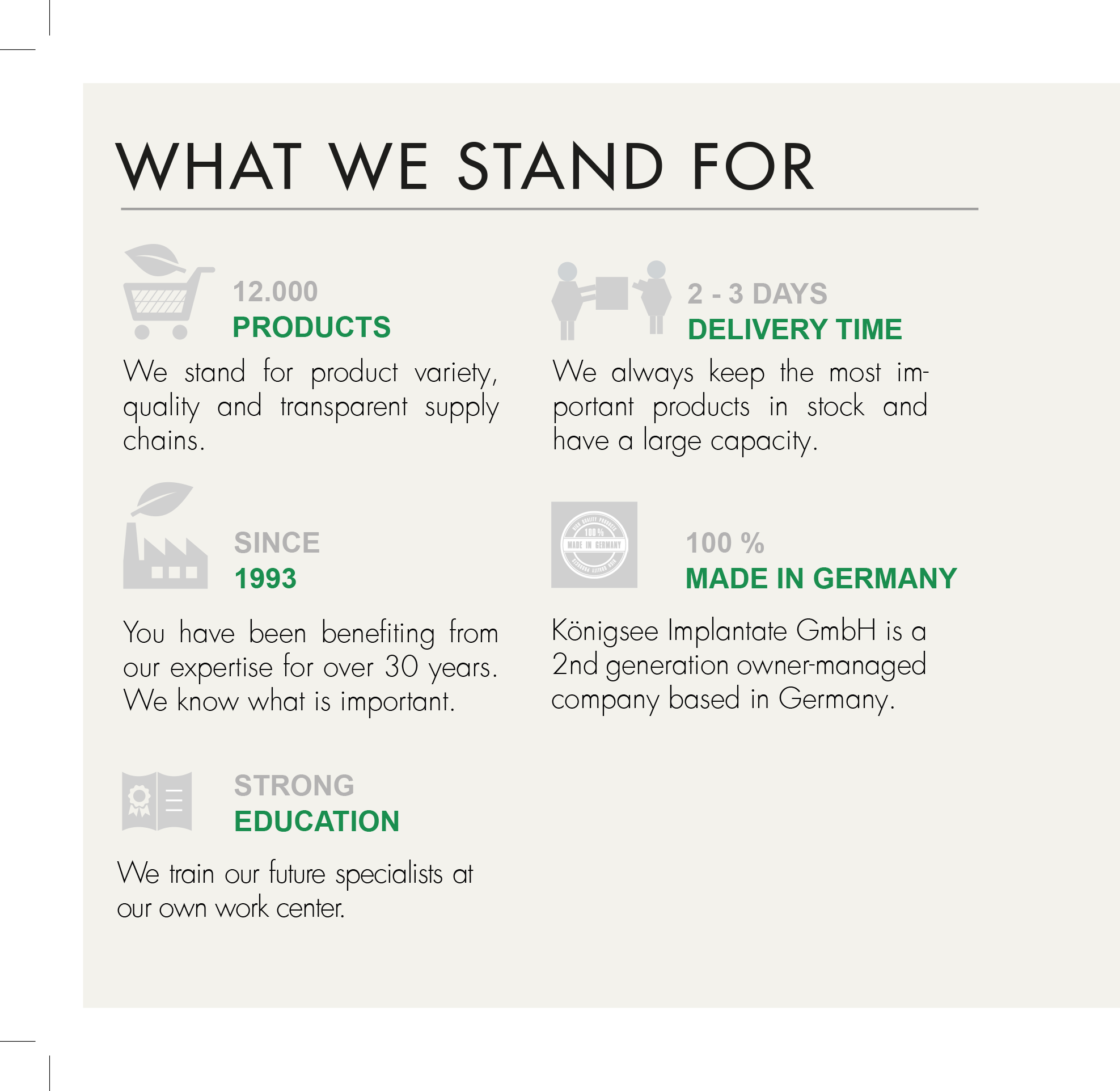
MEDICAL DEVICE REGULATION

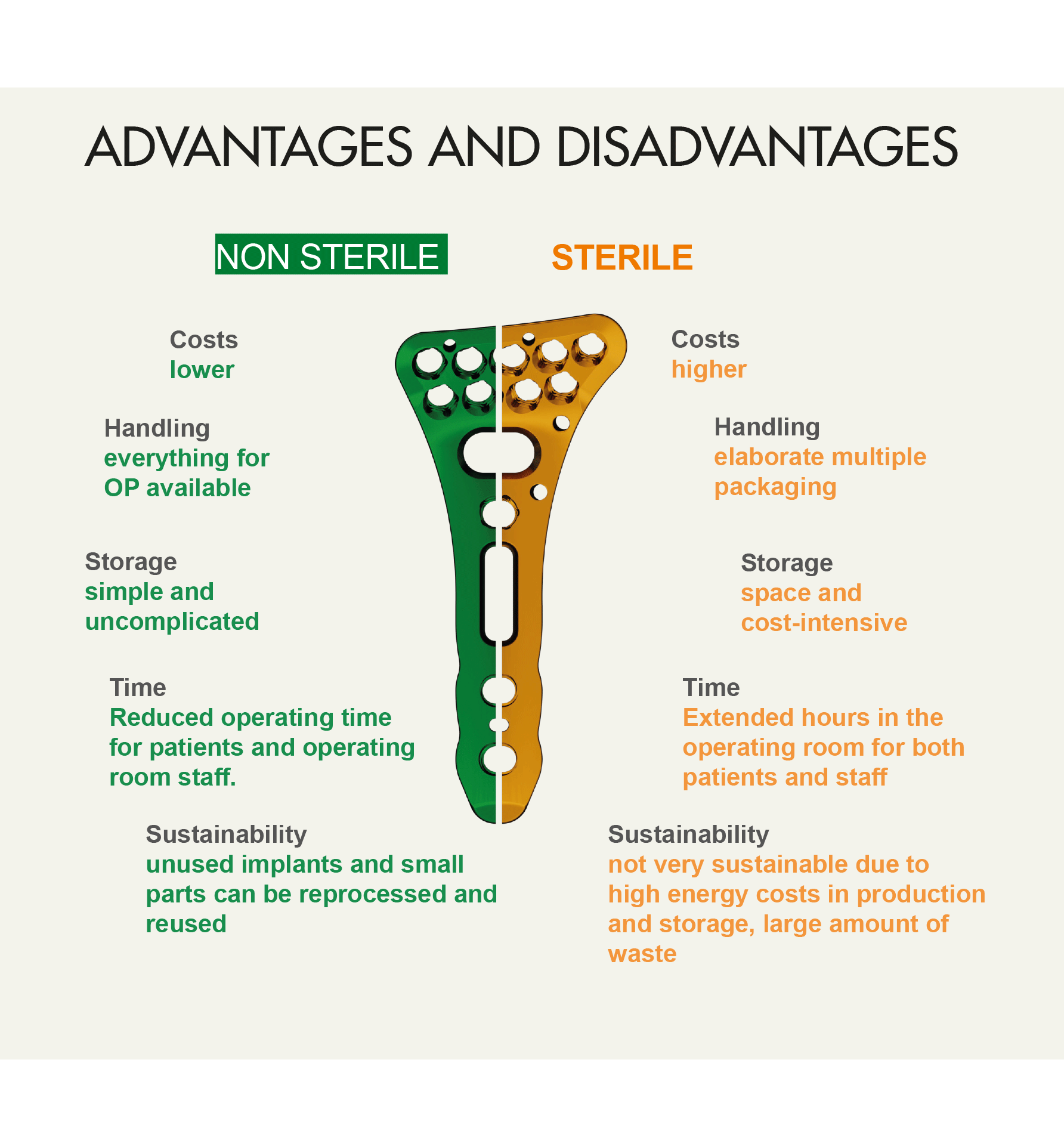
The advantages and disadvantages of non-sterile and sterile packaged implants
Non-sterile packaged implants offer numerous advantages. They are more cost-effective to manufacture, store and use. In contrast to sterile-packed implants, which have to undergo a more complex and energy-intensive external sterilization process, non-sterile-packed implants are more flexible to use.
Make the right decision
Sterile-packed implants require more energy, packaging materials, and generate more waste. The increased consumption of resources has both financial and environmental consequences. Non-sterile surgical kits are a cost-effective and environmentally friendly alternative. Consider these benefits to make an informed decision that balances financial and environmental concerns.